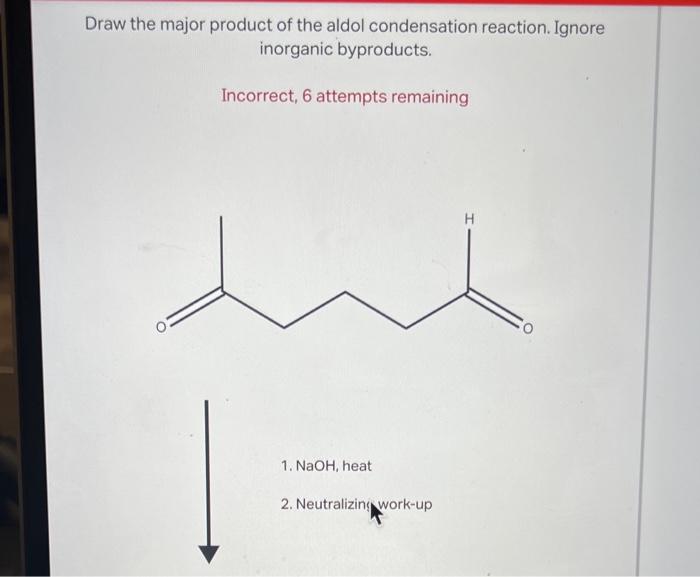
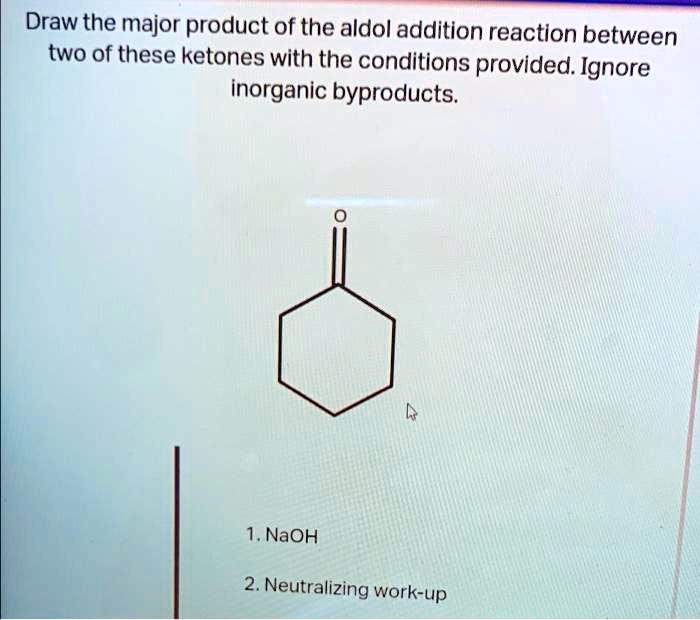
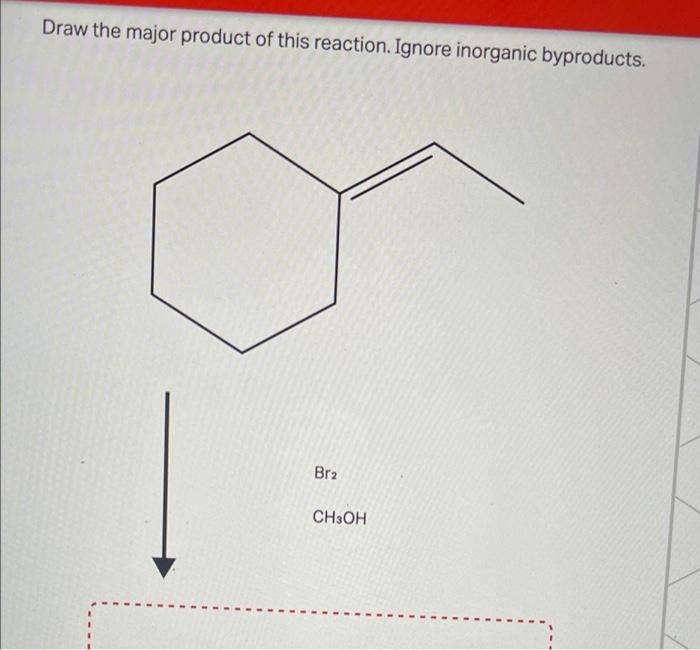
Draw the major product of this reaction. ignore inorganic byproducts – In the realm of organic chemistry, understanding how functional groups interact and transform during reactions is paramount. This exploration delves into a systematic approach to identify the major product of a given reaction, considering regio- and stereoselectivity, while disregarding inorganic byproducts.
By examining the mechanisms and factors that govern these reactions, we uncover the intricacies of predicting and controlling the outcome of organic transformations.
Draw the Major Product of This Reaction
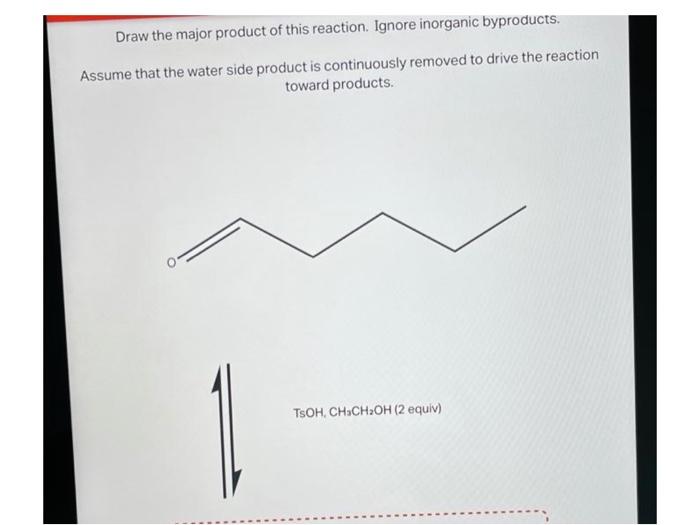
This article will provide a comprehensive guide on how to draw the major product of a given reaction. We will start by identifying the functional groups present in the reactants and explaining how they interact during the reaction. We will then determine the major product based on the reaction mechanism and regio- and stereoselectivity.
Finally, we will consider any inorganic byproducts formed during the reaction and discuss how to minimize their formation.
Identify the Functional Groups
The first step in drawing the major product of a reaction is to identify the functional groups present in the reactants. Functional groups are atoms or groups of atoms that give a molecule its characteristic chemical properties. The most common functional groups include:
- Alkanes
- Alkenes
- Alkynes
- Alcohols
- Aldehydes
- Ketones
- Carboxylic acids
- Amines
- Amides
Once you have identified the functional groups present in the reactants, you can begin to think about how they will interact during the reaction.
Explain the Different Functional Groups Present in the Reactants
The different functional groups present in the reactants will interact in different ways during the reaction. The most common types of reactions include:
- Addition reactions
- Elimination reactions
- Substitution reactions
- Rearrangement reactions
The type of reaction that occurs will depend on the functional groups present in the reactants and the reaction conditions.
Describe How These Functional Groups Interact During the Reaction
The functional groups present in the reactants will interact in different ways during the reaction. The most common types of interactions include:
- Nucleophilic attack
- Electrophilic attack
- Radical reactions
- Pericyclic reactions
The type of interaction that occurs will depend on the functional groups present in the reactants and the reaction conditions.
Determine the Major Product
Once you have identified the functional groups present in the reactants and explained how they will interact during the reaction, you can begin to determine the major product. The major product is the product that is formed in the greatest amount.
The major product can be determined based on the reaction mechanism and regio- and stereoselectivity.
Explain the Mechanism of the Reaction, Draw the major product of this reaction. ignore inorganic byproducts
The mechanism of a reaction is the step-by-step process by which the reactants are converted into products. The mechanism of a reaction can be determined by using a variety of techniques, including:
- Mass spectrometry
- Nuclear magnetic resonance spectroscopy
- Infrared spectroscopy
- Ultraviolet spectroscopy
The mechanism of a reaction can be used to predict the major product.
Discuss the Regio- and Stereoselectivity of the Reaction
The regio- and stereoselectivity of a reaction refers to the preference for one regioisomer or stereoisomer over another. Regioselectivity refers to the preference for one regioisomer over another, while stereoselectivity refers to the preference for one stereoisomer over another. The regio- and stereoselectivity of a reaction can be determined by using a variety of techniques, including:
- NMR spectroscopy
- X-ray crystallography
- Computational chemistry
The regio- and stereoselectivity of a reaction can be used to predict the major product.
Predict the Major Product Based on the Reaction Mechanism
The major product of a reaction can be predicted based on the reaction mechanism. The reaction mechanism can be used to identify the intermediate products and the transition state of the reaction. The major product is the product that is formed from the most stable intermediate product.
Consider Stereochemistry
If applicable, the stereochemistry of the reactants and products should be considered. Stereochemistry refers to the three-dimensional arrangement of atoms in a molecule. The stereochemistry of a molecule can be determined by using a variety of techniques, including:
- NMR spectroscopy
- X-ray crystallography
- Computational chemistry
The stereochemistry of a molecule can be used to predict the major product of a reaction.
Explain How the Stereochemistry Affects the Reaction Outcome
The stereochemistry of the reactants and products can affect the outcome of a reaction. The stereochemistry of a molecule can affect the rate of reaction, the regioselectivity of the reaction, and the stereoselectivity of the reaction.
Analyze Byproducts
In addition to the major product, a reaction may also produce one or more byproducts. Byproducts are products that are formed in a reaction but are not the desired product. Byproducts can be formed in a variety of ways, including:
- Side reactions
- Unwanted reactions
- Decomposition reactions
Byproducts can be a problem because they can reduce the yield of the desired product. The formation of byproducts can be minimized by using a variety of techniques, including:
- Using the correct reaction conditions
- Using a catalyst
- Using a protecting group
Identify Any Inorganic Byproducts Formed During the Reaction
In addition to organic byproducts, a reaction may also produce one or more inorganic byproducts. Inorganic byproducts are products that do not contain any carbon atoms. Inorganic byproducts can be formed in a variety of ways, including:
- Neutralization reactions
- Precipitation reactions
- Redox reactions
Inorganic byproducts can be a problem because they can contaminate the desired product. The formation of inorganic byproducts can be minimized by using a variety of techniques, including:
- Using the correct reaction conditions
- Using a catalyst
- Using a chelating agent
Provide Examples
The following table provides some examples of reactions that follow the pattern described in this article.
| Reaction | Reactants | Products | Reaction Conditions |
|---|---|---|---|
| Addition reaction | Alkene + Electrophile | Alkyl halide | Polar solvent |
| Elimination reaction | Alkyl halide + Base | Alkene + Nucleophile | Nonpolar solvent |
| Substitution reaction | Alkyl halide + Nucleophile | Alkyl halide + Nucleophile | Polar solvent |
| Rearrangement reaction | Alkyl halide + Heat | Alkene | High temperature |
Query Resolution: Draw The Major Product Of This Reaction. Ignore Inorganic Byproducts
What is the significance of identifying the major product in a reaction?
Determining the major product is crucial for predicting the outcome of a reaction and designing synthetic pathways. It allows chemists to optimize reaction conditions and minimize the formation of undesired byproducts.
How does stereochemistry influence the major product?
Stereochemistry plays a vital role in determining the major product, especially in reactions involving chiral molecules. The orientation of functional groups in space can lead to the formation of different stereoisomers, each with unique properties.