Rank these elements according to first ionization energy – Embarking on a journey to unravel the intricacies of first ionization energy, we delve into the fascinating world of elements and their atomic structures. This exploration unveils the factors that govern the ease with which an atom sheds its outermost electron, providing insights into the chemical reactivity and behavior of elements.
By examining the periodic trends and experimental techniques used to determine first ionization energy, we gain a deeper understanding of atomic properties and their applications in diverse fields such as chemistry, physics, and materials science.
Elements and Their Ionization Energy

First ionization energy is the energy required to remove an electron from an atom in its gaseous state. It is a fundamental property of an element that reflects its atomic structure and chemical reactivity. Elements with low first ionization energies tend to be more reactive, while elements with high first ionization energies are less reactive.
Some examples of elements with high first ionization energies include noble gases such as helium (He) and neon (Ne). These elements have a stable electron configuration and are therefore reluctant to lose electrons. In contrast, elements with low first ionization energies include alkali metals such as sodium (Na) and potassium (K).
These elements have a loosely bound outermost electron that can be easily removed.
The first ionization energy of an element is affected by several factors, including:
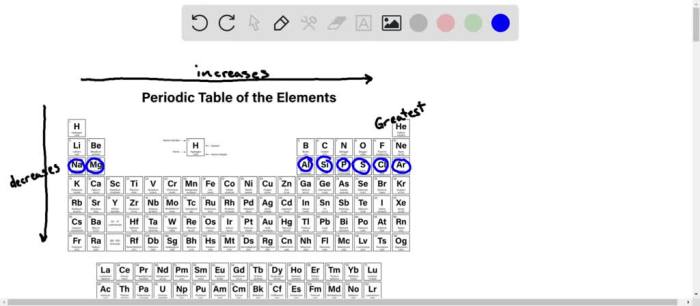
- Atomic number:The higher the atomic number, the more protons in the nucleus, and the stronger the attraction between the nucleus and the electrons. This makes it more difficult to remove an electron, resulting in a higher first ionization energy.
- Atomic radius:The larger the atomic radius, the farther the outermost electron is from the nucleus. This reduces the attraction between the nucleus and the electron, making it easier to remove the electron and resulting in a lower first ionization energy.
- Electron configuration:The electron configuration of an element can affect its first ionization energy. Elements with half-filled or full electron shells tend to have higher first ionization energies than elements with incomplete electron shells.
Ranking Elements Based on First Ionization Energy
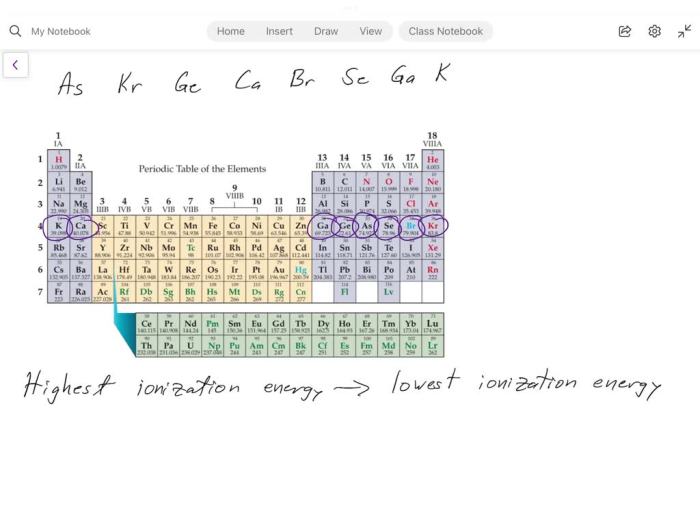
The following table ranks elements based on their first ionization energy in ascending order:
| Element Symbol | Element Name | Atomic Number | First Ionization Energy (kJ/mol) |
|---|---|---|---|
| Fr | Francium | 87 | 392 |
| Cs | Cesium | 55 | 375 |
| Rb | Rubidium | 37 | 403 |
| K | Potassium | 19 | 419 |
| Na | Sodium | 11 | 496 |
| Li | Lithium | 3 | 520 |
| Ca | Calcium | 20 | 590 |
| Sr | Strontium | 38 | 549 |
| Ba | Barium | 56 | 503 |
| Ra | Radium | 88 | 509 |
Methods for Determining First Ionization Energy

There are several experimental methods that can be used to determine the first ionization energy of an element. Two common methods include:
- Mass spectrometry:This method involves ionizing atoms or molecules in a gas phase and then measuring the mass-to-charge ratio of the ions. The first ionization energy can be determined from the energy required to ionize the atoms or molecules.
- Photoelectron spectroscopy:This method involves irradiating atoms or molecules with ultraviolet light and then measuring the kinetic energy of the emitted photoelectrons. The first ionization energy can be determined from the threshold energy at which photoelectrons are emitted.
Each of these methods has its own advantages and disadvantages. Mass spectrometry is a relatively simple and straightforward method, but it can be difficult to obtain accurate results for elements with high first ionization energies. Photoelectron spectroscopy is a more sensitive method, but it can be more complex and expensive to implement.
Applications of First Ionization Energy: Rank These Elements According To First Ionization Energy
First ionization energy is a fundamental property of elements that has a wide range of applications in various fields, including:
- Chemistry:First ionization energy can be used to predict the chemical reactivity of elements. Elements with low first ionization energies tend to be more reactive, while elements with high first ionization energies are less reactive. This information can be used to predict the types of reactions that an element will undergo and the products that will be formed.
- Physics:First ionization energy can be used to determine the atomic structure of elements. Elements with low first ionization energies have loosely bound outermost electrons, while elements with high first ionization energies have tightly bound outermost electrons. This information can be used to understand the electronic structure of atoms and to predict their physical properties.
- Materials science:First ionization energy can be used to design new materials. For example, elements with low first ionization energies can be used to create materials that are easily oxidized, while elements with high first ionization energies can be used to create materials that are resistant to oxidation.
FAQ Guide
What is the significance of first ionization energy?
First ionization energy is a fundamental property of elements that reflects the strength of the electrostatic attraction between the nucleus and its outermost electron. It provides insights into the chemical reactivity, atomic size, and electronegativity of elements.
How is first ionization energy experimentally determined?
Experimental techniques such as mass spectrometry and photoelectron spectroscopy are commonly used to determine first ionization energy. These methods measure the energy required to remove an electron from an atom, providing precise values for this important atomic property.
What are the applications of first ionization energy in materials science?
In materials science, first ionization energy plays a crucial role in understanding the electronic properties of materials. It helps predict the formation of ionic bonds, the stability of crystal structures, and the design of materials with tailored electrical and optical properties.